TheraCyte™ 植入式細胞裝置
TheraCyte™ 植入式細胞裝置
TheraCyte Implantable Cell Device
Cell Encapsulation Device
-封裝細胞治療
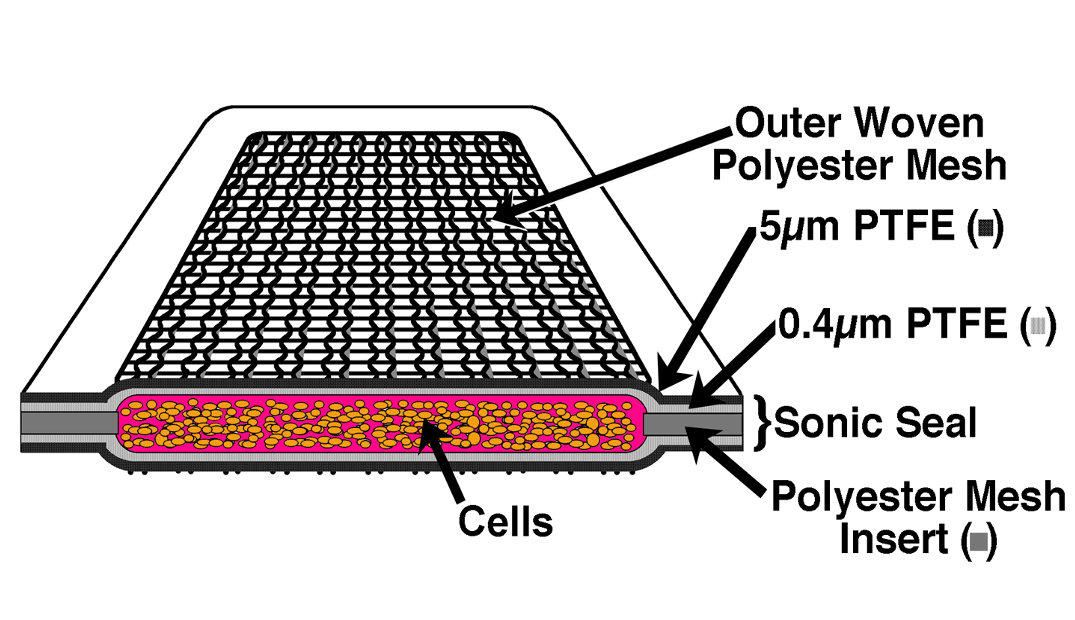
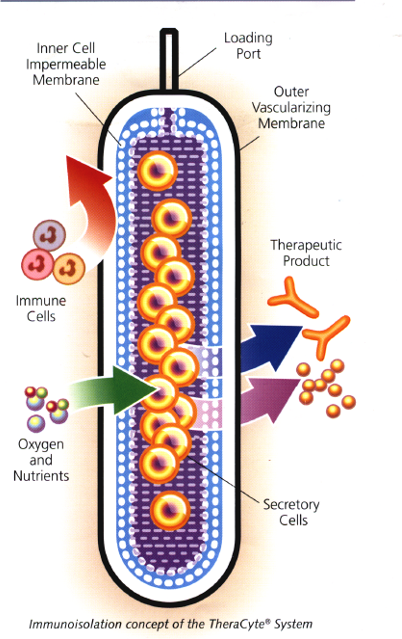
用於封裝和移植細胞的TheraCyte™系統是基於薄膜的聚合物腔室。
它是由生物相容性膜製成的,可保護異體細胞免受受體排斥,
當植入皮下時,可誘導靠近膜的毛細血管生長。
這种血管化功能可提供豐富的血液供應,以滋養膜內的組織,
幫助植入的細胞與宿主溝通,並確保快速吸收治療分子。

※產品優點※
- 無需免疫抑制即可確保移植細胞的安全
- 允許在一個過程中輕鬆植入所有細胞
- 可以通過移除設備來終止藥物/蛋白質評估
- 通過拔出設備執行完全去除細胞的功能,可顯著提高安全性
- 通過正常的宿主血液供應來滋養細胞
※應用領域※
- 糖尿病
- 基因治療
- 治療性蛋白質輸送
- 蛋白質發現
- 免疫學研究
- 抗體遞送
- 細胞移植
- 細胞分化
- 細胞因子治療
- 疼痛管理
- 免疫療法
- 癌症治療
- 體內診斷
- 連續蛋白質輸送
1.浸泡裝置(wetting and sterilization)
2.填充細胞(loading)
3.裝置密封(sealing)
3.植入動物體內
於小鼠/大鼠的應用:
植入位置:
公鼠-副睪脂肪(epidyimal fat pad)
母鼠-卵巢脂肪(ovarian fat pad)
背部皮下(dorsal subcutaneous)
腹部皮下(ventral subcutaneous)
Insulin-producing cells from adult human bone marrow mesenchymal stromal cells could control chemically induced diabetes in dogs: a preliminary study.
Gabr MM, Zakaria MM, Refaie AF, Ismail AM, Khater SM, Ashamallah SA, Azzam MM, Ghoneim MA. Cell Transplantation 1-11. DOI: 10.1177/0963689718759913
Functional Beta Cell Mass from Device-Encapsulated hESC-Derived Pancreatic Endoderm Achieving Metabolic Control.
Robert T, De Mesmaeker I, Stange GM, Suenens KG, Ling Z, Kroon EJ, Pipeleers DG. j.stemcr.2018.01.040. doi.org/10.1016.
3D Printed porous polyamide macrocapsule combined with alginate microcapsules for safer cell-based therapies.
Saenz del Burgo L, Ciriza J, Espona-Noguera A, Illa X, Cabruja E, Orive G, Hernandez RM, Villa R, Pedraz JL, Alvarez M. Scientific Reports (2018) 8:8512. DOI:10.1038/s41598-018-26869-5.
Stem Cell Therapies for Treating Diabetes: Progress and Remaining Challenges.
Sneddon JB, Tang Q, Stock P, Bluestone JA, Roy S, Desai T, Hebrok M. j.stem.2018.05.016. https://doi.org/10.1016.
Allogeneic Ovarian Tissue Encapsulated in Synthetic Poly (ethylene glycol)-Vinyl Sulfone (PEG-VS) and TheraCyte Immunoisolates Allogeneic Ovarian Tissue and Restores Endocrine Function in Ovariectomized Mice.
David A, Day JR, Cichon A, Lefferts A, Cascalho M, Shikanov A. Tissue Engineering Part A Vol 22: S150-S150.
Colony Stimulating Factor-1 Receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates.
Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, et al. Nature materials 16 (6): 671-680. doi:10.1038/nmat4866.
Islet Encapsulation: Physiological Possibilities and Limitations.
Korsgren O. Diabetes 2017 Jul; 66(7): 1748-1754. https://doi.org/10.2337/db17-0065.
Pig-to-Primate Xenotransplantation: Past, Present, and Future.
Liu Z, Hu W, He T, Dai Y, Hara H, Bottino R, Cooper DKC, Cai Z, Mou L. Cell Transplant. 2017 Jun; 26(6): 925–947. doi: 10.3727/096368917X694859
CXCL12 Modulation of Localized Immune Responses to Subcutaneous Islet Macrocapsulation. [abstract].
Penson M, Sremac M, Sirbulescu R, Brauns T, Harrington F, Poznansky M. Am J Transplant. 2017; 17 (suppl 3).
Localized Tolerance and Development of an Alternative Transplant Site to Treat Type 1 Diabetes.
Skoumal, MJ. PhD Dissertation, University of Michigan. ORCID ID: 0000-0001-6993-7369.
Pancreatic islet macroencapsulation using microwell porous membranes.
Skrzypek K, Nibbelink MG, vanLente J, Buitinga M, Engelse MA, deKoning EJP, Karperien M, vanApeldoorn A, Stamatialis D. Scientific Reports volume 7, Article number: 9186(2017)
Considerations for Successful Encapsulated Beta-Cell Therapy.
Thanos CG, Gaglia JL, Pagliuca FW. Cell Therapy, Molecular and Translational Medicine, DF Emerich and G Orive (eds). DOI 10.1007/978-3-319-57153-9_2.
Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted.
Vaithilingam V, Evans MDM, Lewy DM, Bean PA, Bal S, Tuch BE Scientific Reports volume 7, Article number: 10059(2017). doi:10.1038/s41598-017-10359-1.
73 Sox 10+ adult stem cells contribute to biomaterial encapsulation and microvascularization.
Wang D, Wang A, Wu F, Qiu X, Li Y, Chu J, Huang W-C, Xu K, Gong X, Li S. Sci Rep. 2017; 7: 40295. doi: 10.1038/srep40295.
The possible nomenclature of encapsulated products [abstract].
Wani TA, Masoodi FA, Wani IA. Food Chem. 2017 Nov 1;234:119-120. doi:10.1016/j.foodchem.2017.04.121.
Robust, nanofiber-enabled hydrogel devices for islet encapsulation and delivery,
An D, Ma M. Front. Bioeng. Biotechnol. Conference Abstract: 10th World Biomaterials Congress. doi: 10.3389/conf.FBIOE.2016.01.02609.
Survival of encapsulated islets: more than a membrane story.
Barkai U, Rotem A, deVos P. World J Transplant 2016 March 24; 6(1): 69-90. ISSN 2220-3230.
Hypothyroidism Impairs Human Stem Cell-Derived Pancreatic Progenitor Cell Maturation in Mice.
Bruin JE, Saber N, O’Dwyer S, Fox JK, Mojibian M, Arora P, Rezania A, Kieffer TJ. Diabetes 2016;65:1297–1309. DOI: 10.2337/db15-1439.
Inflammasome components ASC and AIM2 modulate the acute phase of biomaterial implant-induced foreign body responses.
Christo SN, Diener KR, Manavis J, Grimbaldeston MA, Bachhuka A, Vasilev K, Hayball JD. Sci Rep. 2016; 6: 20635.
Progress in Clinical Encapsulated Islet Xenotransplantation.
Cooper DKC, Matsumoto S, Abalovich A, Itoh T, Mourad NI, Gianello PR, Wolf E, Cozzi E. Transplantation. 2016 Nov; 100(11): 2301–2308. doi: 10.1097/TP.0000000000001371.
Beta-cell replacement sources for type 1 diabetes: a focus on pancreatic ductal cells.
Corritore E, Lee Y-S, Sokal EM, Lysy PA. Ther Adv Endocrinol Metab 2016, Vol. 7(4) 182–199. DOI: 10.1177/ 2042018816652050.
Immunoisolation to prevent tissue graft rejection: current knowledge and future use.
David A, Day J, Shikanov A. Exp Biol Med (Maywood). 2016 May; 241(9): 955–961. doi: 10.1177/1535370216647129.
Assessment of Immune Isolation of Allogeneic Mouse Pancreatic Progenitor Cells by a Macroencapsulation Device.
Faleo G, Lee K, Nguyen V, Tang Q. World J Diabetes. Nov 15, 2016;7(19): 523-533. doi: 10.4239/wjd.v7.i19.523.
Implanting 1-1B4 human beta-cell pseudoislets improves glycaemic control in diabetic severe combined immune deficient mice.
Green AD,Vasu S, McClenaghan NH, Flatt PR. World J Diabetes.Nov 15, 2016;7(19): 523-533. doi: 10.4239/wjd.v7.i19.523.
Progress and challenges of the bioartificial pancreas.
Hwang PTJ, Shah DK, Garcia JA, Bae CY, Lim D-J, Huiszoon RC, Alexander GC, Jun H-W. Nano Convergence20163:28. https://doi.org/10.1186/s40580-016-0088-4.
Nanomaterials and Regenerative Medicine [textbook].
Lin Y, Gong T (editors). IAPC Publishing, Zagreb Croatia, 2016.
Cell therapies for pancreatic beta-cell replenishment.
Okere B, Lucaccioni L, Dominici M, Lughetti L. Italian Journal of Pediatrics 2016 42:62. https://doi.org/10.1186/s13052-016-0273-4.
Concise Review: Markers for Assessing Human Stem Cell-Derived Implants as B-cell Replacement in Type 1 Diabetes.
Pipeleers D, Robert T, DeMesmaeker I, Ling Z. Stem Cells Translational Medicine AlphaMed Press 1066-5099/2016. http://dx.doi.org/10.5966/sctm.2015-0187.
Progress and Challenges in macroencapsulation approaches for Type 1 diabetes (T1D) Treatment: Cells, Biomaterials, and Devices.
Song S and Roy S. Biotechnol Bioeng. 2016 Jul; 113(7): 1381–1402. doi: 10.1002/bit.25895.
Long term Glycemic Control Using Polymer Encapsulated, Human Stem-Cell Derived B-cells in Immune Competent Mice.
Vegas A, Veiseh O, Gurtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan et al.
Nat Med. 2016 March ; 22(3): 306–311. doi:10.1038/nm.4030.
Quantitative Characterization of Collagen in the Fibrotic Capsule Surrounding Implanted Polymeric Microparticles through Second Harmonic Generation Imaging. Akilbekova D, Bratlie KM. PLoS ONE 10(6): e0130386. doi:10.1371/journal.pone.0130386.
Host response to biomaterials: the impact of host response on biomaterial selection.
Badylak, SF. New York, NY: Academic Press, 2015. 470 pp.
Pancreatic tissue transplanted in TheraCyte encapsulation devices are protected and prevent hyperglycemia in a mouse model of immune-mediated diabetes.
Boettler T, Schneider D, Cheng Y, Kadoya K, von Herrath M. Cell Transplantation 08/2015; 25(3). DOI: 10.3727/096368915X688920.
Innate Immunity and Biomaterials at the Nexus: Friends or Foes?
Christo SN, Diener KR, Bachhuka A, Vasilev K, Hayball JD.
BioMed Research International Volume 2015, Article ID 342304, 23 pages. http://dx.doi.org/10.1155/2015/342304.
Bioengineered stem cells as an alternative for islet cell transplantation.
Moore SJ, Gala-Lopez BL, Pepper AR, Pawlick RL, Shapiro AM J. World J Transplant.Mar 24, 2015;5(1): 1-10.
Polycaprolactone Thin-Film Micro- and Nanoporous Cell-Encapsulation Devices.
Nyitray CE, Chang R, Faleo G, Lance KD, Bernards DA, Tang Q, Desai TA.
ACS Nano, 2015, 9 (6), pp 5675–5682. DOI: 10.1021/acsnano.5b00679.
Macroencapsulation of Pancreatic Progenitors–A New Era in Diabetes Therapy?
Polidori GP. 2015, Vol. 1 No. 1: 5. DOI: 10.21767/2472-1964.100005.
Development of an encapsulated stem cell-based therapy for diabetes.
Tomei AA, Villa C, Ricordi C. Expert Opinion on Biological Therapy 15:9, 1321-1336. DOI: 10.1517/14712598.2015.1055242.
Minireview: Directed Differentiation and Encapsulation of Islet B-Cells–Recent Advances and Future Considerations.
Tse HM, Kozlovskaya V, Kharlampieva E, Hunter CS.
Molecular Endocrinology October 2015, 29(10):1388–1399. doi: 10.1210/me.2015-1085.
Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates.
Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, et al.
Nat Mater. 2015 Jun; 14(6): 643–651. doi:10.1038/nmat4290.
Treatment of diabetes with encapsulated pig islets: an update on current developments.
Zhu H, Lu L, Liu X-Y, Yu L, Lyu Y, Wang B. Zhejiang Univ. Sci. B (2015) 16: https://doi.org/10.1631/jzus.B1400310.
Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape.
Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Stem Cell Research (2014) 12, 807–814. www.elsevier.com/locate/scr
Islet and Stem Cell Encapsulation for Clinical Transplantation
Krishnan R, Alexander M, Robles L, Foster 3rd CE, Lakey JRT
Rev Diabet Stud. 2014 Spring; 11(1): 84–101. doi: 10.1900/RDS.2014.11.84.
Composition and Function of Macro-Encapsulated Human Embryonic Stem Cell-Derived Implants: Comparison with Clinical Human Islet Cell Grafts.
Motte E, Szepessy E, Suenens K, Stange G, Pipeleers D.
AJP Endocrinology and Metabolism 09/2014; 307(9). DOI:10.1152/ajpendo.00219.2014.
Current Status of Islet Encapsulation.
Robles L, Storrs R, Lamb M, Alexander M, Lakey JRT.
Cell Transplantation Vol. 23, pp. 1321–1348, 2014. DOI: http://dx.doi.org/10.3727/096368913X670949.
Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution.
Scharp DW, Marchetti P. Advanced Drug Delivery Reviews 67–68 (2014) 35–73. www.elsevier.com/locate/addr
Review: Macro-Encapsulation of Islets in Polyvinyl Alcohol Hydrogel [abstract].
Sumi S, Yanai G, Qi M, Sakata N, Qi Z, Yang K, Shirouzu Y, Hiura A, Gu Y, Inoue K.
Med. Bio. Eng. 34(3): 204-210. doi: 10.5405/jmbe.1579.
Review: B-cell regeneration and differentiation: how close are we to the “holy grail”?
Tan G, Elefanty AG, Stanley EG. Molecular Endocrinology 2014 Dec;53(3):R119-29. doi: 10.1530/JME-14-0188.
Enrichment of Human Embryonic Stem Cell-Derived NKX6.1-Expressing Pancreatic Progenitor Cells Accelerates the Maturation of Insulin-Secreting Cells in Vivo. Rezania A, deBruin EC, Xu J, Narayan K, Kieffer TJ. Diabetologia 06/2013; 56(9). DOI:10.1007/s00125-013-2955-4.
Review: Macro-Encapsulation of Islets in Polyvinyl Alcohol Hydrogel.
Sumi S, Yanai G, Qi M, Sakata N, Qi Z, Yang K, Shirouzu Y, Hiura A, Gu Y, Inoue K. Med. Bio. Eng. 34(3): 204-210. doi: 10.5405/jmbe.1579.
The TheraCyte Device Protects Against Islet Allograft Rejection in Immunized Hosts.
Kumagai-Braesch M, Jacobsonb S, Moria H, Jiaa X, Tibella A. Cell Transplantation 10/2012; 22(7). DOI:10.3727/096368912X657486.
Inconsistent formation and nonfunction of insulin-positive cells from pancreatic endoderm derived from human embryonic stem cells in athymic nude rats. Matveyenko AV, Georgia S, Bhushan A, Butler PC.
AJP Endocrinology and Metabolism 299(5),e713-e720, 2010. https://doi.org/10.1152/ajpendo.00279.2010.
Real-time bioluminescence imaging of macroencapsulated fibroblasts reveals allograft protection in rhesus monkeys (Macaca mulatta).
Tarantal AF, Lee CC, Itkin-Ansari P. Transplantation. 2009 Jul 15;88(1):38-41. PubMed PMID: 19584678.
Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies.
Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. Transplantation. 2009 Apr 15;87(7):983-91. PubMed PMID: 19352116; PubMed Central PMCID: PMC2715156.
Treatment of diabetic rats with encapsulated islets. J Cell Mol Med.
Sweet IR, Yanay O, Waldron L, Gilbert M, Fuller JM, Tupling T, Lernmark A, Osborne WR. 2008 Dec;12(6B):2644-50. Epub 2008 Mar 28. PubMed PMID: 18373735.
Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: studies in a rodent model.
Sörenby AK, Kumagai-Braesch M, Sharma A, Hultenby KR, Wernerson AM, Tibell AB. Transplantation. 2008 Jul 27;86(2):364-6. PubMed PMID: 18645504.
Treatment of osteoporosis with TheraCyte-encapsulated parathyroid cells: a study in a rat model.
Chou FF, Huang SC, Chen SS, Wang PW, Huang PH, Lu KY. Osteoporos Int. 2006;17(6):936-41. Epub 2006 Apr 5. PubMed PMID: 16596462.
Soluble factor(s) from bone marrow cells can rescue lethally irradiated mice by protecting endogenous hematopoietic stem cells.
Zhao Y, Zhan Y, Burke KA, Anderson WF. (2005) Exp Hematol. 2005 Apr;33(4):428-34.
Xenotransplantation of neonatal porcine liver cells.
Garkavenko O, Emerich DF, Muzina M, Muzina Z, Vasconcellos AV, Ferguson AB, Cooper IJ, Elliott RB. (2005) Transplant Proc. 2005 Jan-Feb;37(1):477-80.
Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys.
Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, Bambra C. (2005) Transplant Proc. 2005 Jan-Feb;37(1):466-9.
Cell-based gene therapy experiments in murine experimental autoimmune encephalomyelitis.
Louie KA, Weiner LP, Du1 J, Kochounian HH, Fling SP, Wei1 W and McMillan M. (2005) Gene Therapy 2005, Jul;12, 1145-1153. (Featured Article)
Porcine endogenous retroviral nucleic acid in peripheral tissues is associated with migration of porcine cells post islet transplant.
Binette TM, Seeberger KL, Lyon JG, Rajotte RV, Korbutt GS. (2004) Am. J. Transplant. 2004 Jul;4(7):1051-60.
Long-Term Erythropoietin Gene Expression from Transduced Cells in Bioisolator Devices.
Ofer Yanay, Simon C. Barry, Lisa Y. Flint, Margaret Brzezinski, Randall W. Barton, and William R.A. Osborne
Human Gene Therapy, Vol 14, pages1587-1593 (November 20, 2003)
Retroviral packaging cells encapsulated in TheraCyte immunoisolation devices enable long-term in vivo gene delivery.
Anna Krupetsky, Zahida Parveen, Elena Marusich, Adrienne Goodrich, and Ralph Dornburg Frontiers in Bioscience 8, a94-101, May 1, 2003
Survival of pancreatic islet xenografts in NOD mice with the TheraCyte device.
Z. Yanga, M. Chena, L. B. Fialkowa, J. D. Elletta, R. Wua and J. L. Nadler Transplantation Proceedings, Volume 34, Pages 3349-3350, 2002.
Research Sponsored by The Islet Replacement Research Foundation
Survival of macroencapsulated allogeneic parathyroid tissue one year after transplantation in nonimmunosuppressed humans.
Tibell A, Rafael E, Wennberg L, Nordenstrom J, Bergstrom M, Geller RL, Loudovaris T, Johnson RC, Brauker JH, Neuenfeldt S, Wernerson A. Cell Transplant 2001;10(7):591-9
Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo.
Amelia Bartholomew, Sheila Patil, Alastair Mackay, Mary Nelson, Diana Buyaner, Wayne Hardy, Joseph Mosca, Cord Sturgeon, Mandy Siatskas, Nadim Mahmud, Karen Ferrer, Robert Deans, Annemarie Moseley, Ronald Hoffman, and Steven M. Devine Human Gene Therapy (2001). 12:1527–1541
Protection of xenografts by a combination of immunoisolation and a single dose of anti-CD4 antibody.
Mckenzie AW, Georgiou HM, Zhan Y, Brady JL, Lew AM. Cell Transplant 2001 Mar-Apr; 10(2):183-93
Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor.
Trivedi N, Steil GM, Colton CK, Bonner-Weir S and Weir GC. (2000). Cell Transplant. Jan-Feb;9(1):115-24
Longitudinal studies on the microcirculation around the TheraCyte immunoisolation device, using the laser Doppler technique.
Rafael E, Gazelius B, Wu GS and Tibell A. (2000). Cell Transplant. Jan-Feb;9(1):107-13
In vivo evaluation of glucose permeability of an immunoisolation device intended for islet transplantation: a novel application of the microdialysis technique. Rafael E, Wernerson A, Arner P, Wu GS and Tibell A. (1999). Cell Transplant. May-Jun;8(3):317-26
In vivo studies on insulin permeability of an immunoisolation device intended for islet transplantation using the microdialysis technique.
Rafael E, Wernerson A, Arner P and Tibell A. (1999). Eur Surg Res. 31(3):249-58
In vivo delivery of recombinant human growth hormone from genetically engineered human fibroblasts implanted within Baxter immunoisolation devices. Josephs SF, Loudovaris T., Dixit A., Young SK. and Johnson RC. (1999). J. Mol. Med. vol 77, 211-214
Correction of diabetic NOD mice with insulinomas implanted within Baxter immunoisolation devices.
Loudovaris T., Jacobs S., Young S., Maryanov D., Brauker J. and Johnson RC. (1999). J. Mol. Med. vol 77, 219-222
Reversal of hyperglycemia in mice after subcutaneous transplantation of macroencapsulated islets.
Tatarkiewicz K, Hollister-Lock J, Quickel RR, Colton CK, Bonner-Weir S, Weir GC. (1999). Transplantation, Mar 15;67(5):665-71
Immunodominant Minor Histocompatibility Antigen Peptides Recognized By Cytolytic T Lymphocytes Primed by Indirect Presentation.
Nevala WK, Paul C, and Wettstein PJ. (l998). Transplantation, 65: 559-69
Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice.
Suzuki K, Bonner-Weir S, Trivedi N, Yoon KH, Hollister-Lock J, Colton CK, Weir GC. (1998). Transplantation, Jul 15;66(1):21-8
Sustained Expression Of High Levels of Human Factor IIX From Human Cells Implanted Within An Immunoisolation Device in Athymic Rodents.
Brauker, J., Frost, G., Dwarki, V., Carr-Brendel, V., Jasunas, C., Hodgett, D., Stone, W., and Johnson, R.C. (1998). Human Gene Therapy 9:879-888
Use of an Immunoisolation Device for Cell Transplantation and Immunotherapy.
Geller, R.G., Loudovaris, T., Johnson, R.C., and Brauker, J.H. (1997). Ann N Y Acad Sci. Dec 31;831:438-51
Immunoisolation of Tumor Cells: Generation of Anti-Tumor Immunity Through Indirect Presentation Of Antigen.
Geller, R.L., Neuenfeldt, S., Levon, S.A., Maryanov, D.A., Thomas, T.J., and Brauker, J.H. (1997). J. Immunother. 20(2):131-137.
Transplantation of Cells In An Immunoisolation Device For Gene Therapy.
Carr-Brendel, V.E., Geller, R.L., Thomas, T.J., Boggs, D.R., Young, S.K., Crudele, J., Martinson, L.A., Maryanov, D.A., Johnson, R.C., and Brauker, J.H. (1997).
Methods Mol Biol. 63:373-87.
Local Inflammatory Response Around Diffusion Chambers Containing Xenografts: Non-Specific Destruction Of Tissues And Decreased Local Vascularization. Brauker, J., Martinson, L.A., Young, S.K., and Johnson, R.C. (1996). Transplantation, Vol 61: No. 12, 1671-1677
CD4+ T Cell Mediated Destruction of Xenografts Within Cell-impermeable Membranes in the Absence of CD8+ T Cells and B Cells.
Loudovaris, T., Mandel, T.E., and Charlton, B. (1996). Transplantation, 61:1678-1684
Structure and function of macroencapsulated human and rodent pancreatic islets transplanted into nude mice.
Andersson A, Eizirik DL, Bremer C, Johnson RC, Pipeleers DG, Hellerstrom C. (1996). Horm Metab Res. Jun; 28(6):306-9
Neovascularization of Synthetic Membranes Directed By Membrane Microarchitecture.
Brauker, J. H., Carr-Brendel, V. E., Martinson, L.A., Crudele, J., Johnston, W.D., and Johnson, R.C. (1995). J. Biomed. Mat. Res., Vol. 29: 1517-1524
Implantable Biohybrid Artificial Organs. Colton, CK. (1995). Cell Transplantation 4(4):415-436. Review Article. TheraCyte Data, pp 427,432,433


