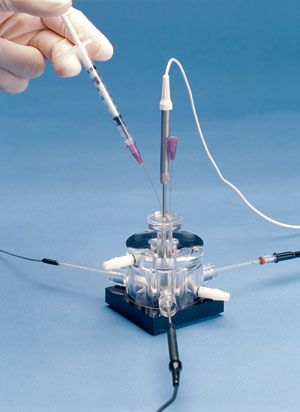
呼吸測量小室(Multi-Port Measurement Chamber)
呼吸測量小室(Multi-Port Measurement Chamber)
型號:NOCHM-4
※產品優點※
密閉室設計大大減少了溶液暴露於空氣中的表面積,可避免測量中的微量誤差。
一個頂部端口和多達三個側面端口配置可提供足夠的空間,方便進行樣品處理和電極操作。
※應用領域※
在受控條件下同時測量培養的細胞,細胞懸浮液或生物培養基中的自由基,
例如NO,H2O2,H2S,O2和其他離子。
主要適用於細胞培養、組織研究,
尤其是粒線體呼吸研究中NO、H2S、CO、O2、HPO、glucose等指標觀察。
如果讓溶液與空氣平衡,則在攪拌條件下將低估溶解在溶液中的NO和其他反應性氣體的量。
腔室由一個緊密配合的蓋組成,通過該蓋可以插入NO探針(ISO-NOP)或其他電極。
當探針就位並且蓋子安裝到腔室時,暴露於空氣的溶液的表面積大大減小。
還提供多達三個可選的側端口,通過它們可以插入氧氣電極*(例如OXELP),
WPI的過氧化氫或KWIK-TIP離子選擇電極,以及WPI的2 mm Dri-Ref™參考電極。
※溫度控制※
通過使用適當的加熱/冷卻循環浴,使水循環通過測量腔的外套,可以方便地控制多端口測量腔的溫度。
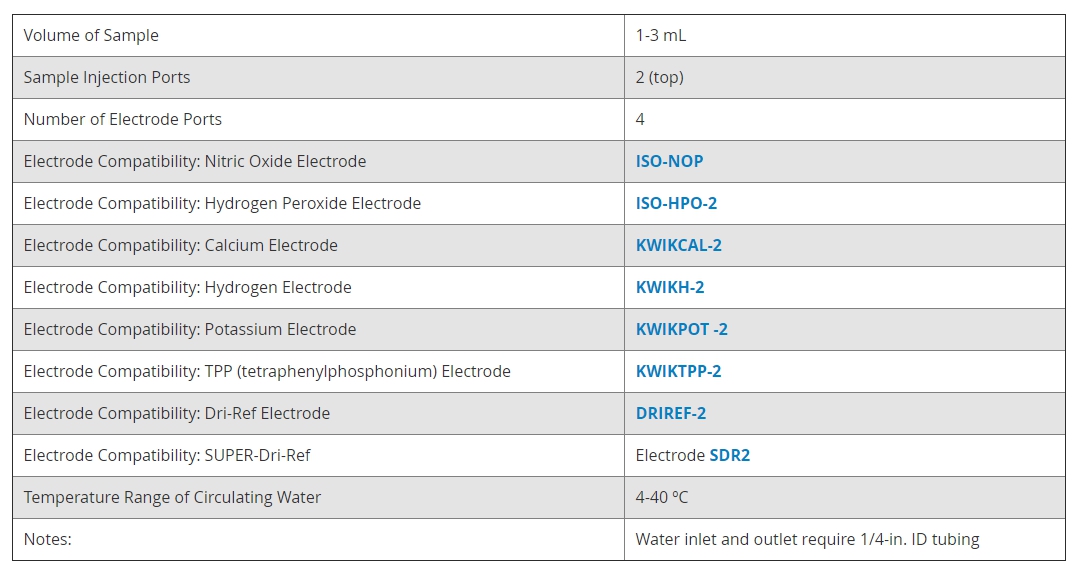
腔室的內部體積(以及因此的樣品體積)可以在1.0 mL至3.0 mL的範圍內連續調節,
適用於大多數細胞懸浮液實驗。



※參考文獻※
Robin, E., Derichard, A., Vallet, B., Hassoun, S. M., & Neviere, R. (n.d.).
Nitric oxide scavenging modulates mitochondrial dysfunction induced by hypoxia/reoxygenation.
Liu, X., El-Mahdy, M. A., Boslett, J., Varadharaj, S., Hemann, C., Abdelghany, T. M., … Zweier, J. L. (2017).
Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall.
Nature Communications, 8, 14807. https://doi.org/10.1038/ncomms14807
Santos, S. S., Jesus, R. L. C., Simões, L. O., Vasconcelos, W. P., Medeiros, I. A., Veras, R. C., … Silva, D. F. (2017).
NO production and potassium channels activation induced by Crotalus durissus cascavella underlie mesenteric artery relaxation.
Toxicon, 133, 10–17. https://doi.org/10.1016/j.toxicon.2017.04.010
Olson, K. R., Gao, Y., DeLeon, E. R., Arif, M., Arif, F., Arora, N., & Straub, K. D. (2017).
Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS).
Redox Biology, 12, 325–339. https://doi.org/10.1016/j.redox.2017.02.021
Zhou, D., Hemann, C., Boslett, J., Luo, A., Zweier, J. L., & Liu, X. (2017).
Oxygen binding and nitric oxide dioxygenase activity of cytoglobin are altered to different extents by cysteine modification.
FEBS Open Bio, 7(6), 845–853. https://doi.org/10.1002/2211-5463.12230
DeLeon, E. R., Gao, Y., Huang, E., Arif, M., Arora, N., Divietro, A., … Olson, K. R. (2016).
A case of mistaken identity: are reactive oxygen species actually reactive sulfide species?
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 310(7), R549–R560. https://doi.org/10.1152/ajpregu.00455.2015
Stephens, R. S., Servinsky, L. E., Rentsendorj, O., Kolb, T. M., Pfeifer, A., & Pearse, D. B. (2014).
Protein kinase G increases antioxidant function in lung microvascular endothelial cells by inhibiting the c-Abl tyrosine kinase.
American Journal of Physiology-Cell Physiology, 306(6), C559–C569. https://doi.org/10.1152/ajpcell.00375.2012
Hemme, D., Veyel, D., Mühlhaus, T., Sommer, F., Jüppner, J., Unger, A.-K., … Schroda, M. (2014).
Systems-Wide Analysis of Acclimation Responses to Long-Term Heat Stress and Recovery in the Photosynthetic Model Organism
Chlamydomonas reinhardtii. The Plant Cell Online, 26(11), 4270–4297. https://doi.org/10.1105/tpc.114.130997
Dantas, B., Ribeiro, T., Assis, V., Furtado, F., Assis, K., Alves, J., … Braga, V. (2014).
Vasorelaxation Induced by a New Naphthoquinone-Oxime is Mediated by NO-sGC-cGMP Pathway. Molecules, 19(7), 9773–9785.
https://doi.org/10.3390/molecules19079773
Liu, X., Tong, J., Zweier, J. R., Follmer, D., Hemann, C., Ismail, R. S., & Zweier, J. L. (2013).
Differences in oxygen-dependent nitric oxide metabolism by cytoglobin and myoglobin account for their differing functional roles.
FEBS Journal, 280(15), 3621–3631. https://doi.org/10.1111/febs.12352
Anidi, I. U., Servinsky, L. E., Rentsendorj, O., Stephens, R. S., Scott, A. L., & Pearse, D. B. (2013).
CD36 and Fyn Kinase Mediate Malaria-Induced Lung Endothelial Barrier Dysfunction in Mice Infected with Plasmodium berghei.
PLoS ONE, 8(8), e71010. https://doi.org/10.1371/journal.pone.0071010
Liu, X., Follmer, D., Zweier, J. R., Huang, X., Hemann, C., Liu, K., … Zweier, J. L. (2012).
Characterization of the Function of Cytoglobin as an Oxygen-Dependent Regulator of Nitric Oxide Concentration.
Biochemistry, 51(25), 5072–5082. https://doi.org/10.1021/bi300291h
Ball, K. A., Nelson, A. W., Foster, D. G., & Poyton, R. O. (2012).
Nitric oxide produced by cytochrome c oxidase helps stabilize HIF-1α in hypoxic mammalian cells.
Biochemical and Biophysical Research Communications, 420(4), 727–732. https://doi.org/10.1016/j.bbrc.2012.03.050
Robin, E., Simerabet, M., Hassoun, S. M., Adamczyk, S., Tavernier, B., Vallet, B., … Lebuffe, G. (2011).
Postconditioning in focal cerebral ischemia: Role of the mitochondrial ATP-dependent potassium channel.
Brain Research, 1375, 137–146. https://doi.org/10.1016/j.brainres.2010.12.054
Talukder, M. A. H., Johnson, W. M., Varadharaj, S., Lian, J., Kearns, P. N., El-Mahdy, M. A., … Zweier, J. L. (2011).
Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice.
American Journal of Physiology-Heart and Circulatory Physiology, 300(1), H388–H396. https://doi.org/10.1152/ajpheart.00868.2010
Ferreira, P. G., Lima, M. A. S. S., Bernedo-Navarro, R. A., Conceição, R. A., Linhares, E., Sawaya, A. C. H. F., … Salgado, I. (2011).
Stimulation of Acidic Reduction of Nitrite to Nitric Oxide by Soybean Phenolics: Possible Relevance to Gastrointestinal Host Defense.
Journal of Agricultural and Food Chemistry, 59(10), 5609–5616. https://doi.org/10.1021/jf201229x
Ball, K. A., Castello, P. R., & Poyton, R. O. (2011).
Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy.
Journal of Photochemistry and Photobiology B: Biology, 102(3), 182–191. https://doi.org/10.1016/j.jphotobiol.2010.12.002
Talukder, M. A. H., Johnson, W. M., Varadharaj, S., Lian, J., Kearns, P. N., El-Mahdy, M. A., … Zweier, J. L. (2011).
Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice.
American Journal of Physiology - Heart and Circulatory Physiology, 300(1).
Robin, E., Derichard, A., Vallet, B., Hassoun, S. M., & Neviere, R. (2011).
Nitric oxide scavenging modulates mitochondrial dysfunction induced by hypoxia/reoxygenation.
Pharmacological Reports : PR, 63(5), 1189–1194. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22180361
Ballot, C., Kluza, J., Lancel, S., Martoriati, A., Hassoun, S. M., Mortier, L., … Marchetti, P. (2010).
Inhibition of mitochondrial respiration mediates apoptosis induced by the anti-tumoral alkaloid lamellarin D. Apoptosis, 15(7), 769–781.
https://doi.org/10.1007/s10495-010-0471-2
Liu, X., El-Sherbiny, G. A., Collard, E., Huang, X., Follmer, D., El-Mahdy, M., & Zweier, J. L. (2010).
Application of carbon fiber composite minielectrodes for measurement of kinetic constants of nitric oxide decay in solution.
Nitric Oxide : Biology and Chemistry, 23(4), 311–318. https://doi.org/10.1016/j.niox.2010.09.002
Liu, Y.-H., & Bian, J.-S. (2010). Bicarbonate-dependent effect of hydrogen sulfide on vascular contractility in rat aortic rings.
American Journal of Physiology-Cell Physiology, 299(4), C866–C872. https://doi.org/10.1152/ajpcell.00105.2010
Neviere, R., Hassoun, S. M., Decoster, B., Bouazza, Y., Montaigne, D., Maréchal, X., … Lancel, S. (2010).
Caspase-dependent protein phosphatase 2A activation contributes to endotoxin-induced cardiomyocyte contractile dysfunction*.
Critical Care Medicine, 38(10), 2031–2036. https://doi.org/10.1097/CCM.0b013e3181eedafb
Stephens, R. S., Rentsendorj, O., Servinsky, L. E., Moldobaeva, A., Damico, R., & Pearse, D. B. (2010).
cGMP increases antioxidant function and attenuates oxidant cell death in mouse lung microvascular endothelial cells by a protein kinase G-dependent mechanism.
American Journal of Physiology-Lung Cellular and Molecular Physiology, 299(3), L323–L333. https://doi.org/10.1152/ajplung.00442.2009
Castera, L., Hatzfeld-Charbonnier, A. S., Ballot, C., Charbonnel, F., Dhuiege, E., Velu, T., … Marchetti, P. (2009).
Apoptosis-related mitochondrial dysfunction defines human monocyte-derived dendritic cells with impaired immuno-stimulatory capacities.
Journal of Cellular and Molecular Medicine, 13(7), 1321–1335. https://doi.org/10.1111/j.1582-4934.2008.00358.x
Rees, M. D., Bottle, S. E., Fairfull-Smith, K. E., Malle, E., Whitelock, J. M., & Davies, M. J. (2009).
Inhibition of myeloperoxidase-mediated hypochlorous acid production by nitroxides.
Biochemical Journal, 421(1), 79–86. https://doi.org/10.1042/BJ20090309
Oliveira, H. C., Saviani, E. E., & Salgado, I. (2009).
NAD(P)H- and superoxide-dependent nitric oxide degradation by rat liver mitochondria.
FEBS Letters, 583(13), 2276–2280. https://doi.org/10.1016/j.febslet.2009.06.012
Presley, T., Vedam, K., Liu, X., Zweier, J. L., & Ilangovan, G. (2009).
Activation of Hsp90/NOS and increased NO generation does not impair mitochondrial respiratory chain by competitive binding at cytochrome C Oxidase in low oxygen concentrations.
Cell Stress and Chaperones, 14(6), 611–627. https://doi.org/10.1007/s12192-009-0114-0
Wulff, A., Oliveira, H. C., Saviani, E. E., & Salgado, I. (2009).
Nitrite reduction and superoxide-dependent nitric oxide degradation by Arabidopsis mitochondria: Influence of external NAD(P)H dehydrogenases and alternative oxidase in the control of nitric oxide levels.
Nitric Oxide, 21(2), 132–139. https://doi.org/10.1016/j.niox.2009.06.003
Pekarova, M., Kralova, J., Kubala, L., Ciz, M., Lojek, A., Gregor, C., & Hrbac, J. (2009).
Continuous electrochemical monitoring of nitric oxide production in murine macrophage cell line RAW 264.7.
Analytical and Bioanalytical Chemistry, 394(5), 1497–1504. https://doi.org/10.1007/s00216-009-2813-x
Castera, L., Hatzfeld-Charbonnier, A. S., Ballot, C., Charbonnel, F., Dhuiege, E., Velu, T., … Marchetti, P. (2009).
Apoptosis-related mitochondrial dysfunction defines human monocyte-derived dendritic cells with impaired immuno-stimulatory capacities.
Journal of Cellular and Molecular Medicine, 13(7), 1321–1335. https://doi.org/10.1111/j.1582-4934.2008.00358.x
Lam, M. A., Pattison, D. I., Bottle, S. E., Keddie, D. J., & Davies, M. J. (2008).
Nitric Oxide and Nitroxides Can Act as Efficient Scavengers of Protein-Derived Free Radicals.
Chemical Research in Toxicology, 21(11), 2111–2119. https://doi.org/10.1021/tx800183t
Liu, X., Yan, Q., Baskerville, K. L., & Zweier, J. L. (2007).
Estimation of Nitric Oxide Concentration in Blood for Different Rates of Generation.
Journal of Biological Chemistry, 282(12), 8831–8836. https://doi.org/10.1074/jbc.M611684200
Hassoun, S. M., Lancel, S., Petillot, P., Decoster, B., Favory, R., Marchetti, P., & Neviere, R. (2006).
Sphingosine impairs mitochondrial function by opening permeability transition pore.
Mitochondrion, 6(3), 149–154. https://doi.org/10.1016/j.mito.2006.05.001
Edwards, J. C., Johnson, M. S., & Taylor, B. L. (2006).
Differentiation between electron transport sensing and proton motive force sensing by the Aer and Tsr receptors for aerotaxis.
Molecular Microbiology, 62(3), 823–837. https://doi.org/10.1111/j.1365-2958.2006.05411.x
Larche, J., Lancel, S., Hassoun, S. M., Favory, R., Decoster, B., Marchetti, P., … Neviere, R. (2006).
Inhibition of Mitochondrial Permeability Transition Prevents Sepsis-Induced Myocardial Dysfunction and Mortality.
Journal of the American College of Cardiology, 48(2), 377–385. https://doi.org/10.1016/j.jacc.2006.02.069
Kramarenko, G. G., Hummel, S. G., Martin, S. M., & Buettner, G. R. (2006).
Ascorbate reacts with singlet oxygen to produce hydrogen peroxide.
Photochemistry and Photobiology, 82(6), 1634–1637. https://doi.org/10.1562/2006-01-12-RN-774
Liu, X., Liu, Q., Gupta, E., Zorko, N., Brownlee, E., & Zweier, J. L. (2005).
Quantitative measurements of NO reaction kinetics with a Clark-type electrode.
Nitric Oxide, 13(1), 68–77. https://doi.org/10.1016/j.niox.2005.04.011
Liu, X., Cheng, C., Zorko, N., Cronin, S., Chen, Y.-R., & Zweier, J. L. (2004).
Biphasic modulation of vascular nitric oxide catabolism by oxygen.
American Journal of Physiology-Heart and Circulatory Physiology, 287(6), H2421–H2426. https://doi.org/10.1152/ajpheart.00487.2004